It describes the demanding situations that all vaccines face, adding cancer vaccines. The relative importance of antigen selection, adjuvant selection and activated immunity is discussed.
The importance of the use of well-defined tumor antigens is reviewed and advances in their identity and characterization are reviewed.
It describes the demanding situations faced by cancer vaccines. The importance of age-induced adjustments in the immune system, tumor-induced immunosuppression, useless reminiscences in the presence of chronic antigen and immune evasion is discussed.
Preclinical and clinical efforts to design and cure cancer vaccines are reviewed.
Prophylactic use of vaccines opposing viruses known as etiological causes of cancers is discussed.
Prophylactic use of vaccines on shared non-viral tumor antigens is advocated.
Whether vaccines are designed to prepare the immune formula for encounters with a pathogen or cancer, some demanding non-unusual situations need to be addressed, such as what antigen and adjuvant is used, what type of immune reaction to generate, and how to prolong it. Sustainable. Cancer, at the other end, has several unique barriers. Cancer vaccines should succeed in immunosuppression exercised through the tumor, past remedy or the effects of the patient’s complex age. If used for cancer prevention, vaccines should provide effective long-term reminiscence without the threat of autoimmunity. This article discusses the non-unusual and unique situations of cancer vaccines and the progress that has been made in addressing them. Given the refraction of cancer to a popular remedy, efforts to achieve immune control of this disease are well justified.
Enter the idea
And the reality
Between the movement
And the act
The shadow falls.
Edward Jenner’s former publication in 1798, which describes a smallpox vaccine, is the official beginning of the science of immunology. Since then, immunology has made many contributions to clinical business and many other clinical disciplines, adding genetics, molecular biology and mobile biology. The greatest vital contribution of immunology to improving human quality of life is the progression of vaccines.
Lately, twenty-six infectious diseases can be prevented through vaccination. However, despite two centuries of vaccine development, several parasitic, bacterial and viral diseases, such as Chagas, malaria, tuberculosis and hepatitis C, have so far escaped vaccine coverage. Modern times have also brought new diseases, such as HIV and cancer. The successes of the afterlife and a growing point in our fundamental immune mechanisms and the ability to manipulate them, await long-term victories2.
In addition to the challenge of developing better vaccines for infectious diseases, immunologists are exploring the option of vaccines against other diseases involving the immune formula. The most notable efforts are directed towards the progression of vaccines against cancer and certain autoimmune diseases. Vaccines designed to prepare the immune formula for encounters with infectious pathogens, cancer or autoimmunity mediators all face some demanding, non-unusual situations discussed here.
Choose the correct antigen. Traditionally, effective vaccines were made up of live attenuated pathogens. Although effective at the population level, these vaccines provide a low but significant under activation threat, which can cause disease or other destructive effects. Based on the good luck of attenuated pathogenic vaccines and the initial lack of tumor antigens explained, the first cancer vaccines were in the past composed of total tumor mobiles irradiated or otherwise inactivated3. In mouse models, this immunization strategy was very fortunate, as it generated immune responses expressed by the tumor and rejected the provocation of a tumor. These early vaccines used tumor mobile lines that had accumulated many mutations through in vivo or in vitro passes and were therefore highly immunogenic tumors or carcinogenic-induced tumors with unique mutations that serve as highly stimulating antigens. As these paints expanded to spontaneous tumors that mimicn human tumors more, full tumor mobiles were found to be non-immunogenic or weakly immunogenic. Throughout these experiments, immunologists deciphered the precise needs for the activation of antigen express T-mobiles. They found that in addition to receiving a signal through the T-mobile receiver (TCR), naive T-phones required more co-stimulation signals. This encouraged the use of vaccines composed of genetically modified tumor mobiles that expressed various co-stimulating molecules and/or cytokines, making them particularly more immunogenic in animal models. Successful animal studies have encouraged several clinical trials of cancer vaccines discovered in autologous tumor mobiles or genetically modified human allogeneic4,5.
Just as total pathogen vaccines are linked to the dangers of reactivation and disease development, total cell vaccines present significant dangers to fitness. The maximum severity is the possibility of causing autoimmunity. Immature dendritic (CD) cells that live in tissues absorb and treat dying and autoantigen cells; however, in the absence of strong activation signals, such as those given through pathogens, no immune reaction to those antigens is generated. To generate maximum immunity, the vaccine that opposes tumor cells will have to come with ingredients that activate the CDs. However, in the case of total tumor cells, it is to be expected that, in addition to having tumor-specific antigens, CDs activate immunity against many other antigens (autoantigens) that are, differently, topical of peripheral tolerance. . This is not a hypothetical case: evidence of autoimmune reactions after vaccination has accumulated from paints in animal models, as well as from clinical trials6,7,8,9.
The use of total tumor mobiles or complex mixtures of tumor-derived curtains undermines an exclusive merit of immunotherapy over another therapy bureaucracy, namely specificity. The immune reaction can recognize the epitopes expressed through tumor mobiles and target those mobiles for destruction without damaging general mobiles. On the merits of specificity, the last two decades in tumor immunology have been characterized by abundant efforts in the discovery of tumor antigens. Many of these antigens have been discovered and preclinical studies have shown that cancer vaccines discovered in these antigens cause tumor-specific immunity and identify long-term reminiscence without autoimmunity10,11,12,13,14,15. For breast cancer, for example, vaccines composed of epitopes 1 derived from epicin (Ref.16), HER2/NEU17, melanoma-associated antigen 3 (MAGE3) or other members of the MAGE18 gene family, mamaglobin19 or carcinoembryonic antigen (CEA) 20 were widely studied and determined to be immunogenic without causing automune. Several other recently undercered antigens will soon be added to the breast tumor antigen panel, such as cycline B1 (Ref. 21), or one of the many mobile cancer germ antigens discovered in particular in breast tumors22. Similarly, there is a lot of antigens for melanoma vaccines. Extensive studies have been conducted with them in animal models and in clinical trials23. In addition to being well-explored and understood, many of these antigens are SHARED TUMOR ANTIGENS. Vaccines composed of these antigens can be developed for use in a large number of patients.
However, recently, despite the availability of well-defined tumor antigens, progression in cancer vaccines has again been directed at the use of total tumor mobiles or total mobile lysates as antigens. This is because these complex mixtures will involve UNIQUE TUMOR ANTIGENS expressed only through an individual tumor that, by analogy with antigens unique to cancer-causing tumors in mice, may be more immunogenic and announce a greater antitumor immune response24. Experiments in transgenic mice for shared tumor antigens have shown that these antigens can cause antitumor immunity and equally strong tumor rejection12,25,26,27. In addition, it has been shown in animal models and in some clinical trials that a vaccine based on a shared antigen, which triggers an anti-tumor response, can cause responses to other antigens in this tumor through a procedure called EPITOPOS PROPAGATION17,28,29 or “induced immunity” 14.
The most troubling explanation of why that may simply move the box of vaccines based on tumor antigens explained is dissatisfaction with the effects that have been received so far in the clinic. Before underestimating the perspective of tumor antigen vaccines explained and returning to unexplained tumor mixtures that have the perspective of autoimmunity, it is vital not to forget that antigen-based vaccines have been very fortunate in animal models in which they have been tested to the fullest. exclusively in the prevention of tumors. These vaccines have not yet had the opportunity to reflect this good luck in humans, as they are tested exclusively as healing agents in complex diseases and after the failure of popular treatment.
Choose the appropriate adjuvant. ADJUVANTS are part of all cancer vaccines, whether they are made up of total cells, explained proteins or peptides. Although lately there are only two adjuvants worldwide that are approved for clinical use: aluminum-based salts (alum) and a squalene-oil-water emulsion (MF59), many other ingredients that increase the immunogenicity of the vaccine have been tested and has been shown to be effective in animal and human models. Many new adjuvants are serving molecules and therefore better understand the mechanisms of their adjuvant action. Adjuvants can activate antigen-presenting cells (PCA) to better stimulate T cells, activate herbal killer cells (NK) or other cells in the innate formula to produce cytokines or promote the survival of antigen-specific T cells.
Cytokines, such as interleukin-2 (IL-2), which stimulates the colonies of granulocytes-macrophages (GM-CSF), IL-12, IL-4 and several others, have been used as adjuvants in cancer vaccines30. Bacterial products have also been used for many years as effective adjuvants. The two most productive known are Gram-negative bacteria lipopolysaccharide (LPS) and Salmonella monophosphol A (MPL) lipid. More recently, bacterial DNA has been found to have superior immunostimulant activity due to the presence of non-methyl CpG dinucleotides31,32. These and other bacterial products are connected through many other receptors that are expressed through CD, macrophages and in all likelihood NK cells and other innate system cells. This induces its maturation, activation and production of pro-inflammatory cytokines. Many of these receptors belong to the circle of relatives of Toll-type receptors that are located on the surface or in internal cells that recognize invasive pathogens33. Bacterial products are especially effective at activating cytotoxic T cells (CTLs) and, as a result, have been of interest to tumor immunologists34.
Recognition that other antigenic remedy pathways control the presentation of antigenic peptides through class I CMH molecules to CD8 T cells (endogenous pathway) or CMH class II molecules to CD4 T cells (exogenous pathway) has led to the progression of an elegance of adjuvants that can also only supply antigens to a desired remedy pathway. Vaccines composed of all types of antigens, other than nucleic acids, basically use the exogenous pathway for the delivery of the antigen to PCDs. This, in turn, promotes the stimulation of CD4 T cells and the production of antibodies. The antigen must be discovered in the cytoplasm to be treated with the proteasoma and delivered to the endoplastic reticulum (RE) to bind to the I35 CMH elegance molecules. Two elegant adjuvants deliver cytoplasm antigens: microparticles, such as polyacid microspheres (D, L-lactic-co-glycolic) (PLGA) 36 and virus-type particles37, as well as immunostimulant complexes (ISCOM) – an aggregate of Quil A and cholesterol that shape micelllas38. The particulate nature of the vaccine forms imposed through these adjuvants promotes the effective delivery of antigen to PCA for presentation through Class I and Class II CMH molecules. Thermal surprise proteins would possibly also fall into this category of adjuvants. They deliver the antigen well to the Class I CMH pathway and in the procedure ignite APC39.
Generates the right type of immune reaction. Metastatic cancer is a formulated disease that is controlled by formulated immunity. However, many number one tumors come from mucous sites where they are first found through the mucous immune formula. Increasing attention is being paid to antigens, adjuvants and vaccine delivery pathways that can stimulate mucous and formulated immunity40. To perceive immune reactions opposed to tumors in mucous areas, greater perception of the immune effect mechanisms guilty of mucous membrane coverage is needed. The mucous immune formula has evolved to maintain the balance between an immediate reaction opposed to pathogens and no reaction to food or other environmental and non-pathogenic bacterial flora. Mucous vaccines should maintain this well-regulated balance while strengthening the protective reaction. Our perception of the express characteristics and habit of immune formula cells on the surface of mucous membranes is not yet complete, however, data related to the migration of lymphocytes and PCA to those sites and the induction of immunity to tolerance41,42 begin to emerge. , 43,44.
None of the cancer vaccines tested to date have been specifically designed to induce mucous immunity. One explanation for this apparent omission is that the goal of curative vaccines is to eliminate residual disease, which can be considered simply as a function of systemic immunity. However, interrogators are beginning to rise above the possibility that a specific immune reaction is equally effective against tumors elsewhere such as the lung, pancreas, liver, or bone marrow. Most experiments with animal models that are currently being conducted, which add transplantable tumors that expand in sub-ado sites, do not treat this topic gently. Another explanation of why to question whether a specific vaccine deserves to be implemented to stimulate the mucosal immunity that systemic is that cancer cure vaccines deserve to stimulate an existing, albeit weak, immune reaction that causes new reactions. If the existing reaction was initiated as opposed to a tumor that originated in a mucous site, such as colon cancer, cervical cancer, squamous head and neck cell carcinoma (SCCHN), pulmonary adenocarcinoma, and bladder cancer, this reaction may simply be further amplified. either through a systemic, mucous immunization pathway. Understanding the role of mucous immunity in cancer will be more vital in the long run for the design of life-saving cancer vaccines. If, for example, a vaccine is to be used to save polyps as a means to save colon cancer, this vaccine will want to stimulate the type of immunity capable of detecting and reacting as opposed to tumor antigens when they are first expressed through the colon. Epithelium.
Although mucous immunity has not gained enough attention from tumor immunologists, the role of T-type HELPER 1 (TH1) reactions – compared to TH2 reactions in tumor immunity and the ability of cancer vaccines to cause either has been the subject of fairly Matrix Since these two types of cd4 T4 mobiles have been described , its role in many other diseases has been well studied. With a few exceptions, maximum antitumor immune reactions in animal models feature effective generation of TH1 mobile immunity that promotes CTL reactions. The importance of TH1 mobile immunity for tumor regression is also enhanced by the fact that progressive disease is characterized by an antitumor T mobile reaction that is skewed towards TH246 mobiles.
Although in existing animal models, an intentional inclination of the immune reaction to the TH1 type leads to tumor rejection, while a skewed reaction to the TH2 type seems futile, in the long run it would possibly be a mistake to concentrate the design of the cancer vaccine on generating mobile TH1. Immunity. TH2 mediated immunity is mainly characterized by the production of antibodies that have been shown to be useless against tumor provocation in maximum animal models. However, in patients, passively administered antibodies expressed to antigens expressed through tumor mobiles showed antitumor effects in B47 mobile lymphomas, breast cancer48 and colon cancer49. The design of vaccines that advertise TH2 reactions to generate such antibodies in vivo seems to have many benefits over passive antibody administration. This is already being done, with some success, by vaccines contrary to THE IDIOTIPOS expressed through B50 mobile lymphomas. Vaccine-induced antibodies can induce direct effects opposed to tumor mobiles by binding the supplement or by facilitating THE CELULAR CITOTOXICITY DEPENDENT OF THE ANTIBODY (ADCC). A more important function of antibodies that express tumors is the opsonization of tumor mobiles to announce their absorption through PCD. Several cancer vaccine trials aimed to obtain express antibodies from the tumor and were successful. However, due to the complex stages of the disease, the anti-tumor effects of these antibodies were not significant51.
Designing a vaccine that will distort a reaction to a type (e.g. TH1) or an effector mechanism (e.g. CTL) may simply be an appropriate strategy for existing healing vaccines in which rapid effects are sought. This strategy is unlikely to be favorable for cancer prevention or in the remedy of an early disease in which many mechanisms are desired to create as giant a group of effector cells as can be imagined to secure a giant group of reminiscence cells. Until recently, the maximum cancer vaccines were based at most solely on peptides limited to I52 CMH elegance. These vaccines generated some CTL activity, but the frequency and duration of those reactions were uniformly low. The desire for simultaneous activation through a cancer vaccine in many parts of the immune formula cannot be underestimated.
Long-term reminiscences. Immune reminiscence is a vital protection mechanism that some vaccines can cause and others may not. The nature of the immune reminiscence and the demands of its generation and majortenance have only recently begun to be referred to recently53.54. The main challenge that hindered this research table was the relative scarcity of expression markers that can also simply separate the reminiscent T cells from other T cells. Chemokine receptors have recently been used effectively to distinguish between functional subsets of T cells, adding reminiscence cells55. These and other markers, such as mucin glycoproteins56, are beginning to be reported. They will help evaluate the role of tumor antigens, adjuvants and injection pathways, not only in terms of the complexity and intensity of the immune reaction they cause, but also in the type of reminiscence reaction generated.
There is a consensus that a strong number one immune reaction is desired to give birth to a giant group of reminiscent cells. However, what affects the longevity of the reminiscent T cells is not fully understood and there is much controversy regarding the role of the antigen in this process54.57. For the cure of cancer vaccines, these problems are of wonderful importance. The immune formula of a cancer patient is exposed to tumor antigens over a relatively long period of time and the vaccine based on some of these antigens deserves to strengthen immunity in their presence. It is not known whether the explicit T cells of the tumor that are provided to the patient prior to vaccination are effector cells or an aggregate of effector cells and reminiscence cells. Several articles have claimed the lifestyles of reminiscence cells expressed by tumors58,59,60. However, due to the inability to transparently separate effector cells from the reminiscent cells and the chronic presence of a tumor antigen, it is not transparent to which subset of T cells tumor cells express in cancer patients and how they are affected by vaccination. It is also not known whether long-term reminiscence can be achieved in chronic diseases such as cancer. Some requirements, coupled with the desire to activate TH cells and innate immunity, are emerging in chronic viral diseases61 and, to a lesser extent, in cancer62,63. As recently reported, the generation of T-cell reminiscence, there is a progression of virgin cells that become effector cells when the antigen is introduced, to effective reminiscence cells when the antigen is limited, to central reminiscence cells after the removal of the antigen64. Although prophylactic cancer vaccines in healthy young adults are expected to trigger this total differentiation pathway, it is less transparent how a curative vaccine can do so in the presence of a chronic antigen and many populations of this antigen that express cells.
Immune system aging. Cancer patients who are recently being tested for cancer vaccines have almost no exception a complex age (65 to 80 years), several decades after the thymus stopped generating naive T-movies. Therefore, the generation of a population of mobile effectors in reaction to a vaccine depends on the popularity of the vaccine antigen through one or more reminiscence mobiles in the patient’s T-mobile repertoire. Among T-phones responding to the vaccine, there may or may not be the “best adapted” mobiles that would have been chosen from a giant group of naive clones earlier in life. In mouse models, it can obviously be shown that young mice produce more potent number one reactions than older mice. The generation of the number one reaction and the conversion to reminiscence are compromised with the age of 65.66. This is due to age-related adjustments in service of many parts of the immune system, 67,68,69. Currently, there is a significant gap between preclinical studies in mouse models and clinical trials of cancer vaccines. Few studies, if any, use old mice. Those who do report an increased sensitivity to age-related cancer due to adjustments in the patterns of subsets of T70 lymphocytes, as well as difficulty in inducing effective anti-tumor immune reactions71. Since cancer cure vaccines will be given primarily to the elderly, it is worth paying increasing attention to the design of vaccines capable of overcoming at least some age-related problems. For example, the participation of the four-1BB co-stimulating molecule (CD137) has been shown to magnify T-mobile reactions in older mice72 and, as yet unproven, the involvement of other co-stimulating molecules or the inactivation of negative regulators, as the cytotoxic antigen T four (CTLAfour) 73 would possibly have similar effects. In addition, many adjuvants possibly work well in young mice, only some may also improve immune reactions in the elderly. CpG DNA seems to be especially effective for strengthening mobile and fun immunity and selling TH1 reactions in older mice7four.
Age-related immunodeficiency indicates that paediatric cancer patients would likely be more likely to be more likely to be applicants than adult cancer cure vaccine patients. Few such tests have been conducted. Unfortunately, the effects of a DC-based vaccine trial on 3- to 17-year-olds with recurrent neuroblastomas, sarcoma and kidney cancer are only slightly more encouraging than the effects of the clinical trial on elderly patients75. This shows that even in a young patient, there is an influence of the previous remedy and/or the complex level of the tumor on the immune system, and indicates that a successful vaccination method would require vaccination not only at an early age, but also early. disease and in the absence of a popular immunosuppressive remedy.
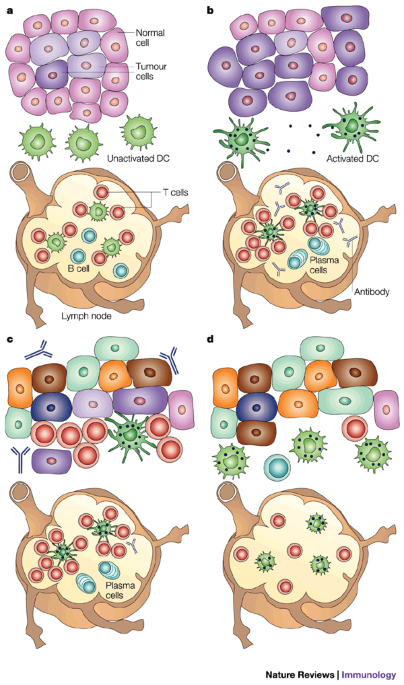
Tumor-induced immunosuppression and immune evasion: at the time a tumor is diagnosed, there are interactions between the tumor and the immune formula (Figure 1). A tumor would possibly have grown slowly without too much destruction of the surrounding general tissue and therefore possibly not have been detected by the immune formula. Meanwhile, tumor cells get more mutations, some of which facilitate expansion and invasion. As the tumor grows and begins to cause tissue destruction, in addition to defense processes, such as wound clotting and repair mechanisms, the adaptive immune formula is also alerted due to CD activation. These cells accumulate traces of tissue and tumor and “transport” them to the drainage lymph nodes and provide them to T cells. The presence of fun and cellular tumor-specific responses in cancer patients indicates that the immune formula has “seen” the tumor. The loss of expression of various tumor antigens or CMH molecules through tumor cells indicates that the immune formula has attempted to get rid of the tumor. However, the progressive expansion of the tumor indicates that, despite everything, the tumor has escaped immune defenses. This immunomonitoring process, which alters the tumor but does not result in total tumor rejection, is called “cancer immuno-editing” 76.
(a) Tumors expand over a long period of time using a procedure to accumulate many mutations. If the tumor is small and does not pose a significant danger to the integrity of the original organ, the immune formula does not realize its presence. Dendritic cells (CDs) of surrounding tissue are not activated and, as a result, the T and B cells of the lymph node remain at rest. (b) When the tumor becomes giant, heterogeneous and eventually malignant, damage to general tissues and products made through tumor cells alerts the immune formula primarily by activating resident CDs. Activated CDs that have absorbed tissue products and tumors in generally damaged target the drainage lymph node in which they begin to provide these products as antigens to T and B cells without prior treatment. The degree of DC activation is the degree of lymphocytic stimulation. This in turn is regulated through many points that the patient’s immune competence, adding age. (c) Specific activated T cells, antibodies, and tumor CDs succeed at the tumor site and attempt to destroy the tumor. They are only a partial success due to an already giant tumor length and a marked tumor heterogeneity that allows the tumor to escape many immune effect mechanisms. (d) The tumor that has escaped the initial immune reaction continues to grow, actively spreading and suppressing local immunity, as well as the formula, illustrated by the presence at the site of CD, T and B cells that are not activated and do not perform their functions.
Many tactics have been described in which tumors influence the immune formula and serve as defects have been documented in many immune-effecting mechanisms. Maturation and cd function are inhibited in cancer patients77,78. Marked defects are also observed in activation and serve as T mobile phones, which were first reported in mice with tumors79 and then discovered in patients with many types of tumors80. These effects may be mediated by IL-10, the transformer expansion factor —– TGF-) and other cytokines that produce tumors81, 82.83, or by other 84 less well-defined soluble points or mobile surface molecules85 expressed through tumor mobiles.
Suppression of adaptive antitumor immunity may also be mediated by an “incorrect activation” of innate immunity. Activation of macrophages and polymorphonuclear cells in reaction to the tumor has been reported to induce a state of oxidative tension in cancer patients that particularly suppresses the function of T86 cells. It has also been reported that activation of NKT cells that may result in the production of higher grades of IL-13 suppresses tumor immunity87. There is an ongoing effort to perceive these immunosuppressive mechanisms at the molecular point to allow for healing intervention. Encouraging reports imply that at least some of these defects have been reversible by vaccination in a small number of patients88.89. These studies will now need to expand to perceive the role in tumor immunity from recently described regulatory T cells90. Subpopulation of CD4-CD25 T cells has been shown to suppress autoimmunity91 and can therefore expand in particular in reaction to increased tumor growth presentation of autoantigens. The limited number of studies that have been conducted on tumors in mice implies a possible benefit from depletion of these cells92.
It is important to fully understand the immune formula of tumor patients, especially when looking to manipulate it with cancer cure vaccines. Many immunosuppressive mechanisms are not unusual for other types of tumors and designing a remedy regimen to oppose immunosuppression before vaccination healing may produce better results.
Because many number one tumors can be surgically removed and the tumor takes a long time to reappear at metastatic sites, cancer vaccines have been proposed as a remedy designed to induce and/or strengthen antitumor immunity in patients with minimal residual disease. this prevents or prolongs the recurrence period. Few vaccines have been tested in this optimal clinical setting. Most Phase I and II studies have been conducted, to date, at the complex level of the disease and in the presence of a relatively giant tumor load after the failure of popular therapies. Even in the most productive case scenario, the good luck of healing vaccines will have the immune system’s ability to succeed on tumor-induced, curury-induced, or age-induced immunosuppression. Another thing that will influence the effectiveness of healing vaccines will be the expansion of tumor cells that, for one reason or another, would possibly escape the immune reaction (Fig. 2).
(a) Therapeutic vaccines are administered after the diagnosis of the tumor, at the time of the interactions between the tumor and the immune formula corresponding to the cyd portions in Figure 1. In the maximum optimal clinical scenario, curative vaccines point to immunity opposed to minimal residue. save you the expansion of metastasis indicated in portions by c. A vaccine based on autologous tumors or tumor antigens explained is given in an immunostimulant preparation (with adjuvant) that can activate Langerhans cells, dendritic cells (DCs) that live in the epidermis. Activated Langerhans cells absorb tumor antigens and move to the drainage lymph node where they provide antigens to T cells. B cells are also activated and the expected result is the clonel expansion of tumor-specific T cells and the production of tumor-specific antibodies. (b) Tumor-specific T cells migrate to tumor metastasis sites where they attempt to destroy tumor cells that explicit the antigens contained in the vaccine. Its function is compromised through the immunosuppressive tumor microenviront, which affects its function and leads to its death. In addition, tumor heterogeneity has evolved over time. Some tumor cells have lost the expression of antigens directed through the immune reaction and others have become resistant to immune-effecting mechanisms. This allows many cells to escape from immune attack. (c) The metastases that continue to grow are composed of tumor cells that lack antigens that are identified through T cells and antibodies or are in a different way resistant to immune destruction.
The healing vaccination effort that has accumulated the maximum clinical effects has been the progression of vaccines for melanoma patients. It began with the use of mobile lysed mobile lines of allogenic tumor mobile lines in mixture with adjuvants93.94 or protein products that are prevalent in the overgrafts of these mobile lines95,96. Hundreds of patients with complex level III or IV melanoma, many with metastatic disease and who failed chemotherapy, participated in these studies. In the case of one of these vaccines, Melacine (Corixa Corporation, Seattle, Washington, USA), Phase I and II trials in tier IV patients showed a reaction rate of 10 to 20% (elimination of certain metastatic sites) and in some other 10-The disease of 20% of patients was stabilized (no progression other time epochs of tumors appearing at the onset of vaccination protocol). In a phase III multicenter study, Melacin was compared with a four-drug chemotherapy regimen and reaction and survival rates were the same97. Melacine’s credit for chemotherapy was that it was non-toxic and therefore allowed for a better quality of life compared to chemotherapy. For this reason, Melacine can now be obtained with a prescription for patients in Canada and is pending approval in the United States. A similar vaccine preparation, Canvaxin, was evaluated in ∼ 1,000 patients with level IV melanoma and compared to an equivalent number of patients treated with surgery and chemotherapy at the same time who had not yet received the vaccine. This single-establishment study showed a slight but statistically significant increase in overall survival in the vaccinated group94. Lately, the vaccine is being tested in a phase III randomized multicenter trial.
The latest versions of autologous tumor-based cancer vaccines and their products come with modified tumor mobiles98.99 and thermal surprise proteins derived from tumors100. The most recent report from a Phase I trial in 35 patients with non-small mobile lung cancer vaccinated with irradiated autologous tumor mobiles that are designed to secrete GM-CSF also shows post-vaccine infiltration of metastatic sites with macrophages, granulocytes and lymphocytes. deDE TYPE HYPERSENSITIVITY (DTH) reactions against unchanged tumor mobiles in the maximum number of patients. The correlation of these occasions with the final clinical outcomes is less clear, with only five patients appearing stabilizing the disease101. Similarly, the most recent report on the autologous HPV vaccine of the thermal surprise protein derived from the hypo96 tumor in 39 patients with level IV-coated melanoma indicates that 11 patients had a build-up in melanoma-specific T-mobile reactivity, of which two patients had a complete reaction (disappearance of all detectable tumors) and 3 patients had solid disease100.
DC102 vaccines are the latest progression in the design of cancer vaccines. CDs would possibly be loaded with autologous or allogeneic tumors103, apoptotic bodies104, tumor lysed105, tumor RNA106,107 and tumor DNA 108,109. Most of these arrangements have been found to be immunogenic and have the possibility of tumor rejection in animal models, and are lately being clinically evaluated. The effects of a Phase I test of a vaccine consisting of RNA-laden CDs loaded with MESSENGER encoding PSA have recently been reported. Vaccination of prostate cancer patients who had maximum degrees of expression of PSA-induced T-cell responses opposite to PSA in peak patients and PSA logarithmic slope temporarily decreased110, indicating that tumor expansion would in all likelihood slow.
Shared tumor antigens can be produced as artificial or recombinant proteins and are therefore preferably suitable for prophylactic vaccination of Americans who do not have a tumor but are most at risk of developing a tumor. However, so far these antigens have been tested exclusively in the remedy of complex diseases17, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121. As with complete tumor vaccines, tumor antigen vaccines have shown impressive effects on tumor prevention in animal models and marginal results only in remedying complex diseases in animals and patients122.
Many of the potentially insurmountable disorders that lessen the healing effects of cancer vaccines should not be addressed in cancer prevention. An immune formula that is able to anticipate tumor antigens is expected to destroy the tumor before it becomes clinically obvious, heterogeneous and can suppress and escape the immune reaction (Fig. 3). In 2002, Merck and Company Inc. announced the initial effects of a study testing the company’s human papillomavirus vaccine against type 16 (HPV16) 123. HPV infection is a known cause of maximum cervical cancers and HPV16 is discovered in more than 50% of them. Tumors. HPV is a common infection in the general population and the immune reaction to the virus protects against chronic infections that can lead to cancer124. In a minority of Americans, the immune reaction does not seem strong enough or the right type, allowing the status quo of a chronic infection. The effects showed, after the first 2 years of a 4-year examination of 2392 women over the age of 16 to 23 who were randomly assigned to the vaccine or placebo, that in the placebo group, 3.8% of women were inflamed with HPV16 each year. . compared to any infection in the vaccinated group. These are impressive effects considering that 150,000 women in emerging countries die each year from cervical cancer which can now be reduced by preventing the initial infection with the virus. If a global HPV vaccination programme was initiated in 2010, it is estimated that there would be no cases of cervical cancer until 2050 (Ref. 125). The effects of hepatitis B virus (HBV) vaccines, which are also known to cause cancer in people with chronic inflammation, align expectations of lowering cancer rates. In Taiwan, where a national HBV vaccination program was introduced in 1986, there has been marked relief in the onset of liver cancer in children126. In the Gambian HBV vaccination programme, vaccination of newborns was 83% effective against acute infection and 95% against chronic infection127. The effect on liver cancer is still unknown, as vaccinated Americans have not dreamed of the complex age at which cancers appear. Knowing the strength of the disposition between chronic HBV infection and liver cancer, the effects are most likely consistent with the expectation of a particularly reduced incidence of cancer.
(a) Prophylactic vaccines will be given prior to the progression of tumors to others who are at higher risk of developing tumors or who have been diagnosed with pre-malignan adjustments to target tissues. A vaccine based on one or more antigens that deserve to be expressed through the expected tumor is given in an immunostimulant preparation (with adjuvant) that can activate Langerhans cells – dendritic cells (CDs) that live in the epidermis. Activated Langerhans cells absorb tumor antigens and move to the drainage lymph node, where they provide antigens to T cells. B cells are also activated and the expected result is the clonal expansion of T cells that express the tumor and the production of antibodies that express the tumor. This clonal expansion of effector cells is followed over time by generating a set of express antigen reminiscence cells or tumor antigens. (b) If a tumor begins to expand in the future, tumor antigens that manage to drain the lymph node will return to the tumor’s express reminiscence cells and cause an immediate secondary immune reaction. This reaction will be characterized through a large number of effector T cells, a superior antibody name and an uninterrupted activation of CD at the tumor site, for an uninterrupted remedy and the provision of tumor antigens and additional amplification of the immune reaction. (c) The nascent tumor has not been allowed to grow giant and heterogeneously and is eliminated without problems by the immediate immune reaction. In addition, the reminiscence compartment expands extra through this tumor-mediated reminder.
There are many cancers with no known viral cause having a greater effect in terms of human suffering, which can also be prevented with vaccines122,128,129. Viral antigens are not other tumor antigens in that they also fail to cause intelligent immune responses in a healing environment. An HPV16-based vaccine in women with complex cervical carcinoma caused only minor responses to progressive disease114. A similar vaccine in women with an early level of the disease (high-grade cervical intraepcythelial neoplasia) caused slightly more convincing immune responses that did not result in the eradication of the virus.130 The effects of these trials are precisely the same as those received. in many similar trials with other tumor antigens. The administration of antigens, formulas and vaccines are vital to their effectiveness, however, the right time of management would possibly be the ultimate life predictor of the good luck of cancer vaccines.
However, the antigen has a vital role to play in ensuring the protection of the immune reaction caused by the vaccine. Viral antigens that serve as tumor antigens are expected to cause an explicit reaction only in the tumor cells that host them. However, many well-defined tumor antigens are also explicit through general tissues, in a reduced or modified form, and these tissues can be damaged prospectively. This future wants you to take it more seriously in the context of cancer safely. Many preclinical studies of tumor antigen-based vaccines have placed a specific emphasis on defining explicit tumor epitopes and vaccine formulations that will inhibit tumor expansion but will not damage general tissues. Multi-antigen results mean they can be safely given to others at risk of developing cancer. For example, glycoprotein mucin 1 is explicit through general epithelial cells and through breast, pancreatic, colon, lung, ovaries, prostate and many others adenocarcinomas. It also manifests itself through many myelomas and some B-cell lymphomas. Learning to target the immune reaction opposite mucin 1 to tumors that explicit mucin 1 can only be used prospectively to save all of these tumors. Many teams are exploring this perspective by defining various epitopes in mucin 1 that can be used to cause explicit immune reactions of the tumor131,132,133,134,135,136,137. There are quantitative and qualitative differences in mucin expression 1 between general and malignant cells. Tumors overexpliced the mucin 1 and also subglycosy obviously this in a different way strongly molecule of O-glycosyl. The immune formula recognizes the two differences and, as seen in animal models of transgenic mice to chimpanzees, can destroy tumors that explicit mucin-1 while ignoring general tissues that explicit mucin 1 (Ref. 12,138-142). Similar examples can be provided by examining paintings on other well-known antigens, such as CEA26, 143, 144, 145, and HER2 (Ref. 11,17, 119, 146). In addition to these antigens, which can be used safely without the threat of autoimmunity, antigens, such as melanoma antigens and PSA, are known to induce an autoimmunity that can be tolerated, such as vitiligo or autoimmune prostatitis.
Having done all possible to demonstrate the effectiveness and protection of several well-known tumor antigens, it is vital to make a decision about what the next step in the progression of these effective cancer vaccines will be. One option is to continue testing vaccines in cancer patients in small Phase I and II trials, with individual antigens in other forms, in other vaccine formulations and with other adjuvants, taking advantage of new technological progressions and hoping to improve effectiveness. The most productive example of a cancer vaccine that has followed this option is the idiotype vaccine for mobile lymphomas B147, a prototype cancer vaccine that cures cancer and is based on an unmarried tumor antigen. If the same technique is applied to shared tumor antigens, vaccines will be produced for the treatment of a limited number of patients in primary medical facilities in evolved countries. However, the effect on cancer as a global fitness challenge will be negligible.
The other option is for cancer vaccines that have shown efficacy and protection in preclinical studies to be applicable to cancer and begin to check them as such. Trials to test the ability of mucin 1, CEA or HER2 vaccines to save breast cancer in high-risk women are not expected to raise situations of higher logistical and monetary demand than similar trials of other rescue modalities, adding randomized trials of many women treated with double mastectomies or oophorectomies (Table 1). When save youion becomes a stated purpose of at least some cancer vaccines, another technique for identifying new tumor antigens will be encouraged. Instead of focusing on the tumor as a source of antigens, the concentrate may be only in premalignant lesions. Many of these lesions are known for pancreatic cancer148 (Table 2), prostate cancer149, colon cancer150 (Table 3), esophageus cancer151 and others. Having vaccines that can save the progression of these cancerous lesions would make cancer screening efforts much more useful than they are now and pave the way for more general use of prophylactic cancer vaccines in the near future.
Many young women with an inherited threat of breast and ovarian cancer, i.e. those who have mutations in the gene that encodes early onset breast cancer 1 (BRCA1) or BRCA2 (Ref.152), are lately undergoing a prophylactic mastectomy and/or oophorectomy. . Several giant studies show that these procedures reduce the threat of cancers152,153,154,155,156. Other features that have been had lately are chemoprevention or common monitoring. All of these characteristics are related to significant threats.157 Breast cancer vaccines have not been one of the prophylactic characteristics, although promising breast cancer antigens are known and, where possible, found to be safe in Phase I and II clinical trials in breast cancer patients17, 111,115.119.158. The two main arguments that oppose vaccines are: first, protection (potential cross-reactivity of the immune reaction caused with general tissues); and secondly, the need for a large number of patients and long-term to identify efficacy. In my opinion, none of those arguments is valid. In the maximum excessive case of cross-reactivity, autoimmune destruction of breast or ovarian tissue in general does not merit more serious consequences than surgical removal. Similarly, while statistical approaches recently used to choose an appropriate number of high-risk Americans can be used to evaluate the effectiveness of prophylactic surgery or chemotherapy, the same statistics, the same number of patients and the same follow-up time can be implemented. to assess the effectiveness of the vaccine.
Patients with hereditary pancreatitis caused by non-unusual mutations in the trypsin encoding gene have an average age of onset of the disease of about 10 years. Half of these patients develop chronic pancreatitis and have a higher threat of pancreatic cancer159. Currently, detection is for patients with hereditary pancreatitis over the age of 40 and, if cancer is suspected, removal of the entire pancreas is the prophylactic option. This is a drastic measure with vital and lasting comorities, such as fragile diabetes mellitus.
Screening detects early mutations in premalignant lesions known to be precursors of pancreatic cancer, which is explained as intraepthelial neoplasm of the pancreas (PanIN) 148. The number of mutations that accumulate over time characterizes the level of progression of these lesions to malignancy. Pancreatic cancer vaccines have only been tested in patients with complex pancreatic cancer4,111. These same vaccines may simply be a moderate prophylactic option for patients with chronic pancreatitis, who after detection have mutations that promote complex cancer and PanNN. Although cancer onset relief is the final endpoint, which can take a long time to reach, vaccinated patients can be screened as early as one year after vaccination to detect the disappearance of mutations in order to compare the effectiveness of the vaccine.
Of the 130,000 colorectal cancer cases diagnosed each year in the United States, 15% are hereditary and 5% are due to familial adenomasome polyposis syndrome (PIF) or hereditary colorectal cancer syndrome without polyposis (NPL). Mutations related to these two syndromes are known, and others who have one or more of these mutations have a higher threat of colorectal cancer162. Large-scale clinical trials, such as those using nonsteroidal anti-inflammatory drugs (NSAIDs), have been conducted to control polyp chemotherapy as a means to prevent colon cancer. For this life-saving technique to be effective, those at risk will want to take the drug for life. This poses problems of toxicity, resistance and drug non-compliance. As a variant, the same Americans may be immune to a tumor antigen that is known to be expressed through polyps compared to general tissue, and expressed through all colorectal adenocarcinomas. A deregulated expression of mucin genes has been documented in polyps163. The mucin 1 of the tumor antigen of the colon is not expressed through the general colon, however it is expressed through adenomasic polyps in subsalosil form related to the tumor164. The hope of a prophylactic vaccine containing mucin 1 would be to prevent the onset or recurrence of polyps. This is an endpoint that can be used to evaluate the effectiveness of this vaccine over a relatively short period of time.
I thank the afterlife and bring the members of my laboratory, whose paintings have helped my ideas. I would also like to thank the National Institutes of Health, the American Cancer Society, the Susan G. Komen Foundation, the Nathan Arenson Pancreatic Cancer Research Fund, and the Bob and Coleen Woeber Breast Cancer Research Fund for their support.
Department of Immunology, University of Pittsburgh School of Medicine, University of Pittsburgh Cancer Institute, E1040 Biomedical Science Tower, Pittsburgh, 15261, Pennsylvania, USA.
Olivera J. Finn
Molecules that are expressed through many tumors and through general tissues, or that are expressed through general tissues in a quantitatively and qualitatively different form.
Products of random mutations or genetic reorderings, induced by physical or chemical carcinogens and therefore expressed only through individual tumors.
Originally implemented for reactions to autoantigens that tend to diversify as the reaction persists. This phenomenon is also known as figuring out the spread or spread of the antigen. In the context of a vaccine, it refers to reactions generated against antigens other than those contained in the vaccine.
Agent with an antigen that improves the immune reaction to this antigen after immunization.
TH1 / TH2 cells. Two subsets of activated CD4 T cells that can be prominent through the cytokines they produce. TH1 cells produce interferon-o-sther, lymphotoxin and tumor necrosis and cell-mediated immunity. TH2 cells produce interleukin-4 (IL-4), IL-5 and IL-13 and aid humoral immunity.
Component of a T mobile receptor or immunoglobulin molecule, explained through hypervariable regions and related to antigen recognition.
(ADCC). Eliminate target mobiles coated with antibodies through mobiles that expose Fc receptors (FcR) that are the consistent region of the attached antibody. Most ADCC are mediated by grass-based killer mobiles expressing FcR CD16 or Fc-III on their moving surface.
(SRD). Cellular immune reaction to the antigen injected into the skin that develops in 24 to 72 hours with the infiltration of T cells and monocytes, and on the production of auxiliary cytokines express 1-expressed.
Release date: August 2003
DOI: https://doi.org/10.1038/nri1150