A vital feature of the intestinal immune formula is its ability to oppose infections and prevent the progression of destructive inflammatory responses to the general microbiota and food antigens.
Intestinal dendritic cell (CD) populations have unique functional properties. They have been concerned about maintaining tolerance to innocent antigens and generating opposing protective immune responses to pathogens.
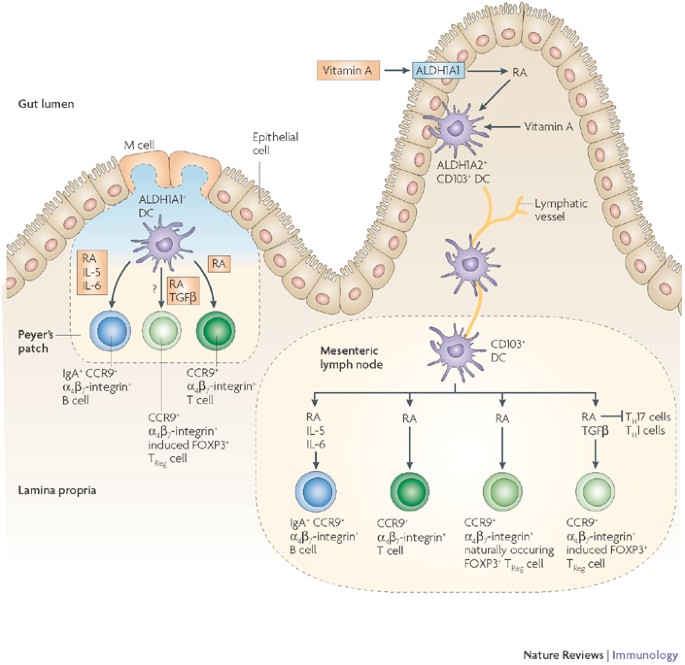
Recent knowledge recommends that intestinal DC would possibly herald the differentiation of P3 regulatory T cells (FOXP3), which regulate the fork head of naive precursors. The expansion process and retinoic acid play a role in this process.
The conditioning of CDs in your local tissue environment may play a role in shaping their role.
The breakdown of intestinal homeostasis can lead to chronic inflammatory bowel disease, adding inflammatory bowel disease. An out-of-place or atypical DC would work, as it would possibly have a role in the pathogeny of inflammatory bowel disease.
The degradation of intestinal homeostasis can lead to chronic inflammatory bowel disease, adding inflammatory bowel disease, celiac disease and allergy. Dendritic mobiles, through their ability to orchestrate protective immunity and immune tolerance in the host, play a key role in the formation of the intestinal immune response. The mechanisms through which dendritic mobiles can respond to environmental signals in the gut and the appropriate immune responses have been misunderstood until recently. Below, we review recent paintings that are beginning to identify the guilty points of dendritic mobile intestinal conditioning that serve as a forthcoming resolution between tolerance and immunity in the gut.
The authors are supported by the Wellcome Trust (F.P.), the European Union (Euro-Thymaide FP6 Integrated Project; LSHB-CT-2003-503410) and the Medical Research Council (J.C.).
Sir William Dunn School of Pathology, University of Oxford, South Parks Road, Oxford, OX1 3RE, United Kingdom
Janine L. Coombes and Fiona Powrie
The authors claim a lack of competitive monetary interests.
(EII). One of the conditions, of unknown etiology, in which the intestinal lining is chronically inflamed. Includes Crohn’s disease and ulcerative colitis.
(pDC). Subset of dendritic cells (CDs) whose microscopic appearance resembles plasmablasts. In humans, these CDs can be derived from negative stem cells in peripheral blood and are the leading manufacturers of interferons I (IFN) in reaction to viral infections. Recent studies have known subsets of TYPE I IFN-producing DDC in mice, which are known through the expression of B220, Ly6C and other markers.
(Microfold Cells). Specialized epithelial cells that release antigens through transpitelial vesicular transport of mild intestinal lymphocytes to intraepitolial lymphocytes and subepithelial lymphoid tissues.
(TReg Cell). A type of CD4 T mobile that is characterized by its expression of the P3 fork head case (FOXP3) and the upper CD25 levels. TReg mobiles can modulate many types of immune responses downwards. The CD4 – CD25 – FOXP3 – TReg mobiles that expand in the scam are exported to the outer edge with their regulatory function as already intact. However, FOXP3 mobile T regulators can also be generated on the outer edge from NAive CD4 T mobiles, for example, through the management of oral antigens or aimed at peptide ligands on in vivo dendritic mobiles.
(T helper 17 cells). Subset of auxiliary CD4 T cells that produce interleukin-17 (IL-17) and are vital in inflammatory and autoimmune diseases. Its generation reaches IL-6, TGF (transforming expansion factor), IL-23 and IL-21, as well as the transcription points MMR (orphaned receiver connected to the retinoic acid receptor) and STAT3 (signal transducer and transcription activator). 3).
Release date: June 2008
DOI: https://doi.org/10.1038/nri2335