Significant progress has been made in the structural determination of IgE proteins and in igE targets and regulation in recent years. This is to feed igE-directed treatments for allergies.
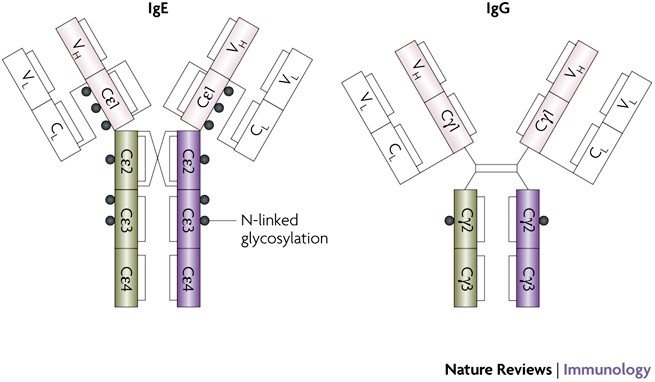
The shape of the IgE molecule differs significantly from that of IgG. X-ray and nuclear magnetic resonance imaging studies have also revealed conformal adjustments that cause the binding of IgE to its high affinity fc-RI receptor (high affinity Fc receptor for IgE) in mast cells and antigen presenting cells, occasions that lead, respectively, to knowledge (and reaction reaction of immediate hypersensitivity reaction) and facilitation of allergens.
Structural knowledge also provides clues about the unique nature of high affinity IgE-FC-IR interaction and involves the ability to block interaction; Validation of IgE as a target is demonstrated through the good luck of omalizumab, an IgE-specific monoclonal antibody, in the treatment of asthma.
The presence of an unusually long extracellular membrane proximal domain in membrane IgE would possibly also be its ability to act as an antigen receptor in B cells and to respond to expressed antigens (allergens).
The trimeric arrangement of type C lectin, the low affinity CD23 IgE receiver, and its susceptibility to excision through ADAM10 (disintegration and metalloproteinase 10) on the mobile surface, provide clues about IgE’s homeostasis mechanism.
CD23 has multiple ligands, adding IgE, CD21 and integrity, allowing you to perform other functions, adding IgE-dependent antigen presentation and cellular cytotoxicity.
IgE is transported to mucous tissue via CD23 and is also synthesized through resident B cells. Its concentration is maintained in the tissue through the number of mast cells that explicit fc-IR in the maximum degrees and the slow rate of dissociation of fc-IRI IgE.
Class substitution in IgE and maturation of antibody affinity occur in mucous tissue, possibly restricting the ability of IgE antibodies to mediate in systemic anaphylaxis.
Several mechanisms manage to suppress the production of IgE synthesis at tolerable points, as well as restrict their anatomical distribution. Some mechanisms work at the smart switching recombination point, others at the survival point of IgE-switched cells.
Sending IgE in any of the instructions through the gastrointestinal epithelium would possibly be related to early allergen awareness. Studies on the early awareness mechanism may recommend tactics to prevent the progression of an allergic disease.
Small inhibitory molecules in the IgE-Fc-IR interaction would possibly update the express antibodies of IgE, however, combining this technique with immunotherapy would possibly be for a more effective healing intervention in allergies and asthma.
The growing epidemic of allergies and asthma is generating greater interest in IgE, a central player in allergic response. IgE activity is related to a protein network; These come with their two main receptors, Fc-IR (an fc receiver of superior affinity for IgE) and CD23, as well as galectin-3 and several co-receivers for CD23, adding CD21 and integrins. Here, we review recent advances in the discovery of the structures of these proteins and their complexes, and in our understanding of how IgE exerts its effects and how its expression is regulated. Emerging data suggest new healing instructions to combat allergic diseases.
We thank the Medical Research Council (UK), Wellcome Trust and Asthma UK for our paintings in this area. We also thank K. Kirwan, A. Davies and R. Beavil for their preparation for the original figures.
Randall Division of Cell – Molecular Biphysics, and MRC – Asthma UK Centre in Allergic Mechanisms of Asthma, King’s College London, New Hunt’s House, Guy’s Campus, London, SE1 1UL, United Kingdom
Hannah J. Gould and Brian J. Sutton
Hannah J. Gould and Brian J. Sutton
(FRETTE). A quantum mechanics procedure through which the excitation power, without photon emission, is transferred from a donor fluorophore to a nearby accepting fluorophore. FRET can be used for intermolecular or intramolecular distances (in diversity from 10 to one hundred degrees).
(EMPD). A domain that provides in the heavy chain of a membrane immunoglobulin, located between the C-terminal domain of the soluble antibody (C-4 in IgE) and the transmembrane sequence.
(APC). The cells that can internalize and treat the antigen then demonstrate fragments of antigenic peptides on their surface, as well as the molecules needed to activate similar lymphocytes.
(ITAM). A short peptide trend containing tyrosine residues that is discovered in the cytoplasmic tail of various signaling-adapting proteins and that recruits proteins involved in the activation of activating signaling proteins. The consensus series is Tyr-X-X- (Leu / Island) -X6-8-Tyr-X-X- (Leu / Island), where X designates any amino acid.
A circle of calcium-dependent protein relatives. The bonding activity of type C cithins is based on the design of the carbohydrate popularity domain (CRD), which is highly preserved in this circle of relatives. Calcium contributes to the structural maintenance of this domain and is essential for its function of binding to carbohydrates.
(FDC). A type of mobile that is found only in the germ centers of lymphoid tissue and presents an antigen for determined B mobiles and provides mandatory survival signals for the maturation of affinity.
A peptide trend composed of the amino acids arginine, glycine and aspartic acid, is not unusual for many ligands that bind to integrins.
The biological and clinical consequences that occur in the first hour of crosslinking IgE complexes with Fc-IR (high affinity Fc receptor for IgE) on the surface of mast cells and/or basophils through allergens. Clinical manifestations are characterized by the specific effects of tissues, adding constriction of giant airlines in asthma and skin reactions of “papules and outbreaks”. Widespread symptoms in several target organs may come with edema and itching .itchy. Systemic manifestations may come with angioedema, hives and, in severe cases, vascular sinking (anaphylaxis).
Clinical manifestations may be measurable (visible) two hours or more after exposure to an allergen, but may also appear much later. These occasions peak 6 to nine hours after exposure to the allergen and disappear within 24 to 48 hours. Reactions are characterized by edema and infiltration of auxiliary T cells 2 and eosinophils. Tissue reactions are characterized by edema, pain, heat and rash (redness). Pulmonary reactions are characterized by narrowing of airlines and hypersecretion of mucus.
(RSE). The procedure by which a gene segment from the variable region of the heavy chain to a segment of a gene from the consistent region of the heavy chain in the explicit heavy chain gene is recombined with a segment of the region’s gene consistently downstream to make explicit a new antibody elegance.
(SHM). The procedure by which point mutations occur in the gene segments of the variable region of the heavy or soft chain, resulting in a substitution in the expressed protein, which would possibly adjust the affinity or specificity of the antigen (or allergen).
The procedure through which the survival and proliferation of B cells is decided based on their affinity for the antigen.
Professional dendritic cells that provide antigens in the skin epidermis.
(FAP). Also known as facilitated allergen presentation, this is the procedure by which CD23 internalizes allergens-IgE and recycles peptides with class II CMH molecules on the moving surface for T-mobile recognition.
The procedure by which an antibody reaction to an antigen epitope leads to the production of express antibodies for other epitopes of the same antigen or for absolutely independent antigen epitopes. These effects of the internalization of the complete antigen and the next demonstrate a diversity of peptides derived from this antigen, which leads to the generation of T-mobiles with other expressions of epitopes. The simultaneous remedy of two unrelated antigens through a mobile antigen presenting can lead to the production of antibodies aimed at opposite to any of the antigens.
Condition, mediated by IgE antibodies, with greater sensitivity to immediate hypersensitivity.
Release date: March 2008
DOI: https://doi.org/10.1038/nri2273